Enabling Cell and Gene Therapy Start Ups to Thrive and Deliver
Director of Sales and Marketing Darrell Tanner reveals how MES and release by exception with POMSnet helps Cell and Gene Therapy company’s delivery right first time
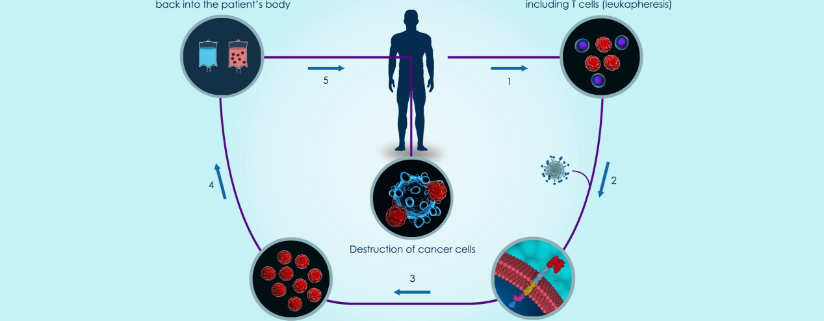
We are seeing personalized medicine formally launch businesses into mainstream life sciences. Cell & Gene Therapy and CRISPR editing technology are integrating into the portfolio offering of many pharma, biotech, and new life science companies. In CAR-T medicine white blood cells and T-cells are extracted from a patient and re-engineered to identify cancer cell surfaces. Timing and coordination are paramount to delivering a potential cure to the patient. Most start-up Cell and Gene Therapy companies are in the throes of Phase 1 and Phase 2 clinical trials and collaborating with contract manufacturing organizations (CMO) to develop and manufacture cures. Pharma and Biotech companies, University Researchers, and life science start-ups are challenged with securing and identifying patient information, donor information, and collection/infusion. The chain-of-identify (CoI) and chain-of-custody (CoC) are strictly enforced in the process to ensure patient safety. To meet FDA record requirements for production many Cell and Gene Therapy companies are seeking out MES electronic batch records for personalized medicine solutions. Pharma and Biotech companies are getting smart and designing in MES electronic batch records from day one of these new processes.
POMSnet MES for regulated industries delivers Material Management, Genealogy, Equipment Tracking (safety hoods, instruments, centrifuges, and more) and Electronic Batch Record execution which help manufacturers deliver cell therapies faster by streamlining the production recipe workflow with a cloud-based manufacturing execution system (MES). It’s simple to understand that cancer patients don’t have much time to wait for therapy records to be manually reviewed and then released. And manufacturers can’t introduce more risk into the system with manual or paper operations. Any part of the supply chain operations that can be automated to process quicker will be a strategic advantage for Cell and Gene Therapy companies. We see personalized medicine companies are setting themselves up with Manufacturing Execution Systems to efficiently manage recipes from the clinical phase trails to full commercial production.
Electronic batch records (EBR) are a strategic area where POMSnet can help Cell and Gene therapy companies manage the risk and ensure right first time to patients while building a competitive advantage. With and MES release-by-exception or review-by-exception functionality companies can immediately adopt the benefits during Phase 1 and Phase 2 clinical trials and beyond. By easily sharing the production records or responding to deviation and alarms in real-time CMO’s can deliver CAR-T and CRISPR therapies exactly to customer specifications. EBR is just one example of how MES can quicken the pace to commercialization.
We hear from customers that our validated cloud-based MES system is ideal fit for controlled environments in Cell and Gene Therapy labs. Customers take advantage of native HTML5 code in POMSnet user screens to allow production on any mobile device with any browser to access the application wireless which reduces infrastructure and maintenance. A cloud-based and HTML5 MES eliminates the physical burdens labs deal with so operators can focus on the engineering of cells and genes first. Other customers are taking advantage of the financial benefits a Software as a Service (SaaS) model offers their budgets by leasing a system rather than capital expenditure. There are tax savings by switching to an expense on cost of goods sold when customers go with our proven MES Software as a Service (SaaS) leader in our industry.
If your company is producing CAR-T or using CRISPR Therapies, contact POMS to learn how implementing a cloud-based MES in phase 2 or phase 3 clinical trials will help you to commercialize.